Australasian MARS – Multicentre Aspiration Risk Study

What is MARS?
MARS is an Australasian multicentre observational cohort study looking at rates of pulmonary aspiration under anaesthesia.
The study aims to assess whether permitting clear fluid drinks until the start of anaesthesia (“Sip Til Send”) is non-inferior compared to advising patients to stop drinking 1 or 2 hours before anaesthesia (restrictive fluid fasting).
Why do we need MARS?
Despite national guidelines advising patients to consume clear fluids until 1 to 2 hours before an anaesthetic, in practice patients often go without fluids for much longer – commonly averaging 7 to 13 hours [Markman 2024]. This can cause thirst, dehydration, heightened anxiety, nausea, and issues with blood sugar management.
Sip Til Send fasting has become an effective and popular strategy to shorten fluid fasting duration before anaesthesia, improving the patient experience and avoiding the problems related to dehydration. Between 2023 and 2025, approximately 50 Australasian hospitals changed their preoperative fasting policies to routinely offer their patients Sip Til Send [Markman 2024].
Pulmonary aspiration under anaesthesia is rare and in most cases the consequences are mild, but it remains an important cause of severe complications during anaesthesia. Studies to date have suggested that Sip Til Send is equally as safe as restrictive fasting with regard to aspiration [Andersson, Marsman, EUROFAST], but we need to study larger populations to be sure of these results.
The MARS project is designed to provide the best evidence yet on the safety of Sip Til Send.
Study design
The MARS project will collect aspiration data from 250,000 adults and children undergoing anaesthesia for elective or emergency procedures in participating hospitals across Australia and New Zealand.
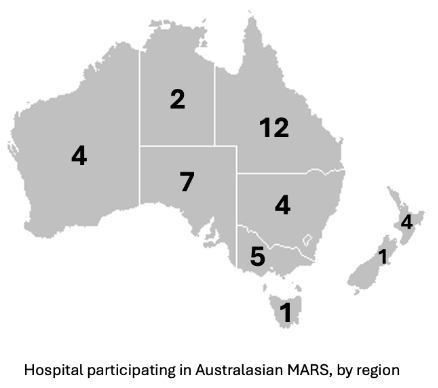
Registered hospitals
As of May 2025, there are 40 hospitals registered for the MARS project, spanning all parts of Australia and New Zealand
Register your hospital for MARS
Each participating hospital has ONE Principal Investigator (PI), who is typically an anaesthetist. The PI will require the support of the hospital’s research governance officer to complete the site specific assessment. Data collection has been designed to be low burden and will only require one individual (the PI, a trainee, or a research co-ordinator).
Why do MARS?
- Each PI gets listed as author on the main publication arising from the study, while associate investigators who have significantly contributed get acknowledged in a list of collaborators.
- Assistance provided by ANZCA trainees may contribute to their Scholar Role requirements (check with local supervisor).
- Data collection phase is low burden when compared to a clinical trial.
All hospitals in Australia and New Zealand are eligible to join MARS, provided the following pre-requisites are met during the data collection phase:
- Your hospital has an actively-promoted aspiration audit that is considered low risk for under-reporting (see below)
- The principal investigator at each hospital must be able to identify the patients involved in reported aspirations, to enable diagnosis verification and the collection of additional data.
Features of an aspiration audit that may minimise under-reporting:
- Well-promoted so that users are aware an audit is active, and why it is important the audit generates accurate data.
- Clear diagnostic criteria for pulmonary aspiration, built into the questionnaire structure
- Reporting system is “low friction” and efficient for the user, and does not require delaying the entry of the report until ALL data are known. This typically consists of the treating clinician being required to enter only the “essential” data usually known early after an aspiration event, followed by a study investigator later completing the case data based off medical records.
Study progress
April 2025
Multicentre ethics approval has been granted by the respective authorities in Australia and New Zealand, and recognised by all hospitals in their respective countries.
40 participating hospitals are listed in these ethics approvals.
Funding has been secured to facilitate the study’s design and implementation.
May 2025
Commencement of local governance approval application process for participating sites.
Study co-ordination
Co-ordinating Centre:
Department of Anaesthesia and Perioperative Medicine – Cairns and Hinterland Hospital and Health Service, Queensland, Australia
Lead Investigators:
Dr Phuong Markman, Staff specialist anaesthetist, Cairns Hospital, Australia

Dr Ruth Blank, Staff specialist anaesthetist, Cairns Hospital, Australia
New Zealand Coordinator:

Dr Alec Beresford, Specialist Anaesthetist – Health NZ Waitaha Canterbury – Christchurch hospital, New Zealand
Supervisor:

Professor David Story. President, ANZCA. Head, Dept of Critical Care, The University of Melbourne.

